When using the latest immunotherapy treatment methods, 'modified' immune cells are introduced into the body in order to attack cancers, tumours and other targets.
Researchers at the Technical University of Munich (TUM) have developed a method for tracking these cells in the body. This new approach offers greater insight into what actually happens in the body and could deepen our understanding of these types of cellular therapies - as well as help make future treatments safer.
What is a T cell?
They are cells in the blood that protect us from infection. T cells are a type of white blood cell also known as T lymphocytes. Lymphocytes fights infectious agents such as viruses and bacteria - also fighting other harmful cells - such as cancer cells. So, from a research perspective it is really helpful to be able to monitor and watch how they respond to different types of disease.
New method for labeling T cells in immunotherapy
When standard treatments for diseases like cancer fail, custom-tailored cell therapies are increasingly becoming a viable option.
A prominent example is known as CAR-T-cell therapy. In this approach, immune cells are taken from the patient and genetically engineered in the lab to carry a receptor that recognises structures specific to the surface of cancer cells. These modified immune cells then multiply in the body and initiate an immune response against the tumour.
Important questions need answers
Physicians could greatly benefit from knowing exactly how these modified immune cells behave in the body. There are important questions such as:
- Do they migrate to where they are needed?
- Do they replicate sufficiently?
- Do they behave unpredictably and,
- in the worst-case scenario, attack healthy tissue (cause autoimmune disease)?
Currently, there are no clinically applicable methods to answer these critical questions.
An artificial receptor and a custom-designed marker
An interdisciplinary team at TUM and the TUM University Hospital has now proposed a solution. In simplified terms, a second artificial receptor is inserted into the modified immune cells. These cells can then be visualised using PET scan imaging with a specially developed, non-toxic radioactive contrast agent. When this so-called radioligand is injected into the body, it binds exclusively to the modified cells and their descendants, making them visible.
Showing how these cells migrate
In the experiments the researchers were able to demonstrate that the modified cells indeed migrated to the affected diseased tissue and proliferated there. They also showed that the radioligand is rapidly excreted via the kidneys, binds exclusively to cells with the artificial receptor, and does not interfere with other processes in the body. Moreover, the study showed that this approach can also be used to monitor gene therapies in which viruses serve as tools to alter genetic information within cells.
A valuable tool
“For several years now, it has been clear that new medical applications like immunotherapies and gene therapies hold tremendous potential,” says Prof. Wolfgang Weber, who led the study. “We believe that we have created a valuable tool that can make such therapies safer by providing better insight into what happens inside the body.” The technique is still in its early stages. Before it can be used in human patients, its safety and efficacy must be verified in clinical trials. Further development toward clinical trial use and commercialization is currently ongoing.
Still, the researchers believe the method can already yield valuable insights for basic research. It is also intended to support animal welfare: If laboratory animals can be continuously monitored during experiments, their numbers could be significantly reduced in the development of new cell and gene therapies.
Notes
The technique relies on artificial proteins with specific binding properties, known as anticalins. These have been developed since the 1990s by Arne Skerra, Professor of Biological Chemistry at TUM and a pioneer in protein engineering. His work led to the creation of an anticalin that binds the ligand DTPA and has now been adapted as part of a cell surface receptor. A team led by Wolfgang Weber, Professor of Nuclear Medicine at TUM University Hospital, used this concept to engineer an artificial gene that causes cells to express the anticalin receptor "DTPA-R" on their surface. The project was spearheaded by Volker Morath and Katja Fritschle from the Department of Nuclear Medicine, who, along with their team, also developed the matching radioligand for DTPA-R: 18F-DTPA. The method was tested on CAR-T cells in collaboration with immunotherapy expert Dirk Busch, Professor of Medical Microbiology, Immunology and Hygiene at TUM.
Prof. Wolfgang Weber
Technical University of Munich
TUM University Hospital
Pic: courtesy of TUM -
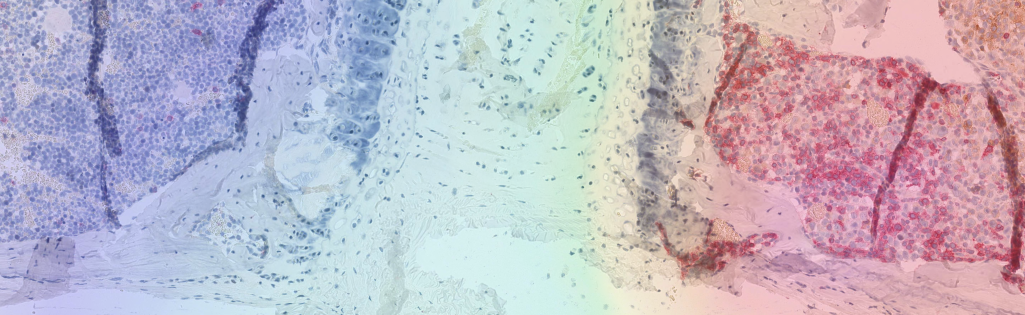
Caption - In the right half of this tissue section, engineered immune cells known as CAR T cells are attacking tumor tissue. A new method makes it possible to visualize them in the body using PET imaging, as shown here in the superimposed image. Copyrights / Free for use in reporting on this research with the copyright noted
Publication:
Volker Morath, Katja Fritschle et al. “PET-based tracking of CAR T cells and viral gene transfer using a cell surface reporter that binds to lanthanide complexes.”Nature Biomedical Engineering (2025). DOI: 10.1038/s41551-025-01415-7
Further information:
- Also contributing significantly to the study was PD Dr. Katja Steiger from the Institute of Pathology at TUM.
- Research into methods for tracking immune cells using imaging techniques is underway in many places. At TUM, for example, the start-up ImuVeo – which also has its roots in the Department of Nuclear Medicine and the Department of Medicine III at TUM University Hospital – has developed a technique that enables immune cell tracking.