Rheumatoid arthritis is a common autoimmune condition resulting in progressive joint damage. This article will be of help to anyone who would like more information about the latest treatments for rheumatoid arthritis.
Rheumatoid Arthritis - Arthritis Consultant Overview
- What is rheumatoid arthritis?
- What are the symptoms of rheumatoid arthritis?
- How is rheumatoid arthritis diagnosed?
- What treatments are there for rheumatoid arthritis?
- How long will I need to remain on treatment?
- What is the outlook?
What is rheumatoid arthritis?
Rheumatoid arthritis is a type of inflammatory arthritis that affects 1% of our population. In keeping with many autoimmune conditions it is more common amongst women. It is thought to arise through a complex interplay of genetic and environmental factors which leads to activation of the immune system and inflammation of the lining of the joint. This process results in redness, warmth, pain and swelling of the joints. The treatments we use in rheumatoid arthritis aim to suppress the inflammation, thus improving symptoms and also crucially preventing progressive damage to the joints. The inflammatory burden in rheumatoid disease is associated with osteoporosis and increased cardiovascular risk, it is therefore important to screen for and address risk factors for these conditions also.
What are the symptoms?
Rheumatoid arthritis can present in different ways but is classically a symmetrical arthritis involving joints on both sides of the body and primarily affecting the wrists and small joints of the hands and feet. Other joints which are commonly affected include the shoulders, knees and ankles. One third of patients present with quite an abrupt or acute onset of disease which is often associated with more generalised features such as fatigue, weight loss and low grade fevers. In others, the arthritis comes on gradually, or in older patients may present with pain and stiffness affecting the hip and shoulder girdle. Less commonly, patients may have involvement of a single joint or inflammatory disease outside the joints.
How is it diagnosed?
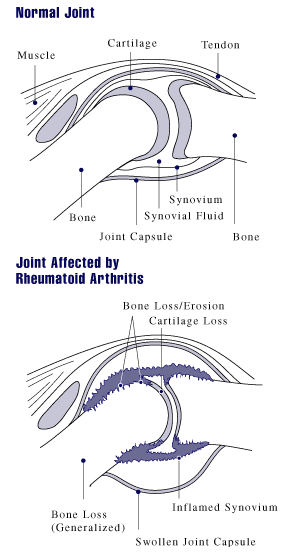
The early diagnosis of rheumatoid arthritis is important: research has shown that early initiation of treatment is more effective in the long-term and reduces the development of progressive joint damage. A diagnosis of rheumatoid arthritis requires review by a Rheumatologist who will take a history, perform a physical examination and request further blood tests, x-rays and may consider referring you for an ultrasound or MRI scan.
Particular blood tests of interest include the Erythrocyte Sedimentation Rate (ESR), C Reactive Protein (CRP), Cyclic Citrullinated Peptide antibodies (CCP) and Rheumatoid Factor (RF). An elevated ESR or CRP is indicative of an inflammatory response which is seen in rheumatoid arthritis. RF is positive in 50% to 80% of rheumatoid arthritis patients but is also found in up to 5% of the population with no disease. It is therefore not a good test in isolation for diagnosing rheumatoid arthritis. CCP is a newer more specific test for rheumatoid arthritis. The presence of RF and CCP suggests the presence of more severe disease.
In early disease, x-ray radiographs of the hands and feet may only show soft tissue swelling or reduced bone mineralisation on either side of the involved joints. In established disease, erosions of the joint may be visible. Ultrasound and MRI scanning allow a more detailed assessment of the joints. Power Doppler Ultrasound has the ability to detect low velocity blood flow in small blood vessels which is often seen in inflammatory joint disease.
What treatments are there?
The treatment of rheumatoid arthritis has progressed dramatically over the last decade. Rheumatoid arthritis remains a spectrum of disease with some patients having very mild symptoms ranging to those with rapidly progressive joint destruction. Once joints have been damaged they do not heal very well, it is therefore important to suppress the inflammation early to limit any damage to the joints. If treatment is started early it is more likely to be effective. In choosing the appropriate treatment we need to balance the disease severity and likelihood of progressive joint damage against the possible side effects of therapy. There are four main groups of drugs used in the treatment of rheumatoid arthritis:
- Non-Steroidal Anti-Inflammatory Drugs
- Corticosteroids
- Disease Modifying Anti-Rheumatic Drugs
- Biologic therapies
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs such as naproxen can help to reduce pain and swelling in rheumatoid arthritis but are unable to control progressive joint destruction. If taken regularly they can cause ulceration of the lining of the stomach and also carry a slightly increased risk of heart attack or stroke. Although these drugs may be needed to control pain in the short term, it is preferable for them to be used on an occasional basis only.
Corticosteroids
Corticosteroids are used to achieve rapid control of inflammation whilst other medications take time to start working. They can be administered as an intra-muscular injection, directly into an inflamed joint or as tablets. Use of joint or muscle injections is preferable as this approach minimises the long-term steroid exposure. However, sometimes patients may require oral prednisolone.
Disease Modifying Anti-Rheumatic Drugs (DMARDs)
DMARDs control the underlying disease process in rheumatoid arthritis and slow or prevent progressive joint damage. They usually take a period of six weeks or more to start working and the dosage of these drugs may need to be increased gradually over time. This group of drugs includes oral DMARDs such as Hydroxychloroquine, Sulphasalazine, Methotrexate and Leflunomide. There is evidence that when these drugs are used in combination they can be more effective in controlling inflammation.
Methotrexate was one of the first drugs to be used in the treatment of rheumatoid arthritis in the 1980s and remains the ‘anchor drug’ both as a treatment alone and in combination with other therapies. Methotrexate is usually given as a once-weekly oral preparation, although in those patients who develop gastro-intestinal side effects it can be administered as a subcutaneous injection. Sometimes patients can feel nauseous or unwell with methotrexate, this usually settles as the body adapts to the medication and it is worth persevering with the therapy if the side effects are not too severe.
Biologic therapies
A new group of disease modifying drugs, referred to as the biologic therapies have had a significant impact on the management of rheumatoid arthritis over the last decade. These are injectable drugs that target a specific protein or molecule involved in the inflammatory process. They are only used if patients have failed treatment with oral DMARDs. The Biologic Drugs include the anti-TNF drugs (infliximab, etanercept, adalimumab, certolizumab pegol and golimumab), Rituximab, Abatacept and Tocilizumab.
The anti-TNF drugs are the first line biologic agents used when conventional DMARDs fail. There are five different anti-TNF drugs available and whilst no ‘head-to-head’ studies have been undertaken they all appear equally efficacious with similar side effect profiles. The choice of which drug to use is largely governed by convenience of administration and the particular characteristics of the patient. Infliximab is given via an intravenous infusion whilst etanercept, certolizumab pegol, adalimumab and golimumab are administered via subcutaneous injections. The long-term safety data for these drugs is good with no increase in the rate of solid malignancies although there is evidence of a slight increase in non-melanoma skin cancers. An important potential side effect of these medications is the risk of infection. Infection risk is greatest in the first six to twelve months of therapy. Where risk of infection arising through use of these drugs is a concern, a choice of an anti-TNF agent with a shorter half-life is preferable. There is an increased risk of tuberculosis arising during treatment with anti-TNF drugs. Screening patients for evidence of previous tuberculosis exposure and treating accordingly can reduce the risk of active tuberculosis. We routinely undertake a chest x-ray and interferon-gamma release assay (blood test) in these patients. We also screen patients for hepatitis B, as there is a risk of fulminant hepatitis developing in the context of active hepatitis B infection. Anti-TNF drugs should not be used in patients with heart failure or a history of de-myelinating disease such as multiple sclerosis.
If despite anti-TNF therapy there is persistent disease activity, an alternative anti-TNF drug can be trialled. However, studies suggest that it may be more effective to start treatment with Rituximab. Rituximab depletes B cells in the peripheral blood. It is administered by two intravenous infusions a fortnight apart and is usually repeated when symptoms return which can be from six months to three years after the initial treatment. Rituximab carries a similar risk of infection to the anti-TNF drugs. This is greatest in the three-month period after an infusion and may be linked to low immunoglobulin G levels, which are routinely checked prior to each infusion. There is no increased risk of tuberculosis reactivation, although screening for hepatitis B infection is advised.
Alternative biologic agents for the treatment of rheumatoid arthritis include tocilizumab and abatacept. Tocilizumab blocks the IL-6 receptor and is administered intravenously once a month. Abatacept is also administered intravenously and blocks T cell co-stimulation. Tocilizumab and abatacept have a similar risk of infection to the anti-TNF drugs. Tocilizumab has been associated with bowel perforation and should therefore be avoided if there is a history of diverticulitis or stomach ulcers. Abatacept should be used with caution in patients with chronic obstructive pulmonary disease in light of an increase in pulmonary complications in this population.
Do biologic drugs affect vaccination?
Due to the increased risk of infection with rheumatoid disease and use of biologic therapies, it is advisable to have the pneumovax and influenza vaccinations, ideally prior to starting treatment. However, the vaccine response on anti-TNF and even rituximab does appear to be sufficient and vaccination is therefore advisable if prior immunisation cannot be achieved. Live vaccines such as yellow fever are not recommended whilst using biologic drugs due to a theoretical risk of developing infection.
How long will I need to remain on treatment?
For the majority of patients the treatments used are long-term or life-long therapies. After a period of stability we may try to reduce DMARD or biologic therapy. During this time you will need to be monitored closely for reactivation of the disease. If the arthritis does flare it may not be possible to control it with the same dosages or medications previously employed. Research has shown that you are more likely to achieve drug free remission in the future if treatment is started early.
What is the outlook?
The outlook for rheumatoid arthritis is good. A large amount of research has been conducted into rheumatoid arthritis and we now have a wide range of effective therapies. There are also new treatments on the horizon. It is important to be reviewed by your specialist regularly and to aim towards tight control of your disease.
For further information on the author of this article, Consultant Rheumatologist, Dr Pamela Mangat, please click here.
Non-steroidal anti-inflammatory drugs. A group of drugs that provide pain relief and reduce inflammation.
Full medical glossary




